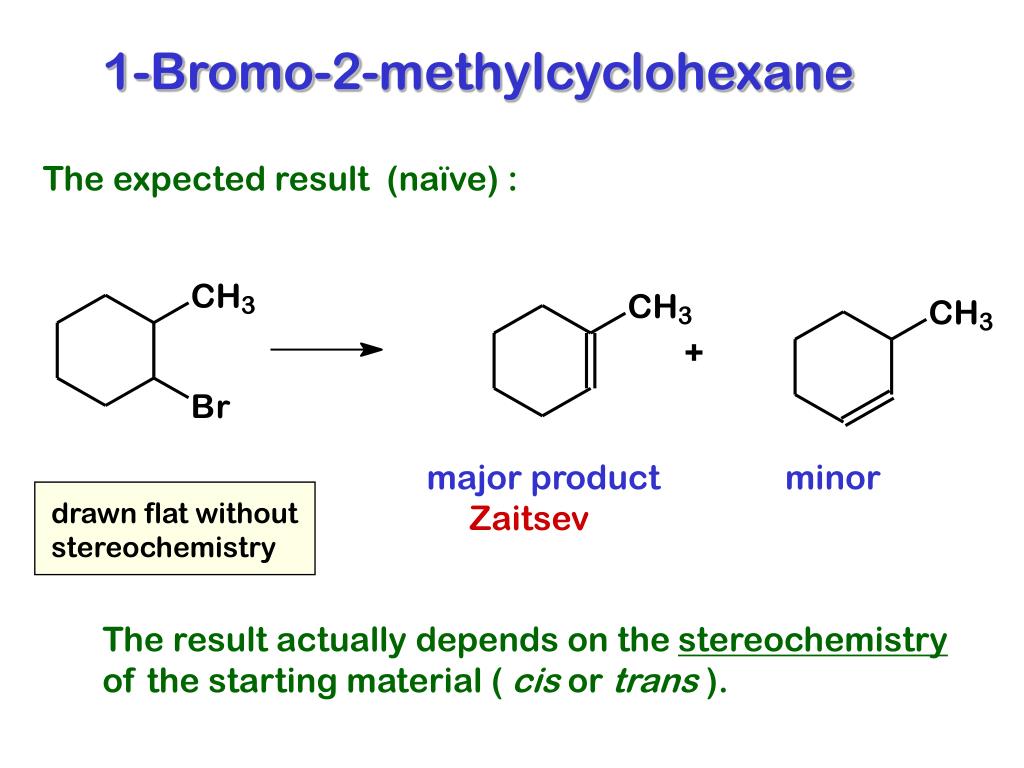
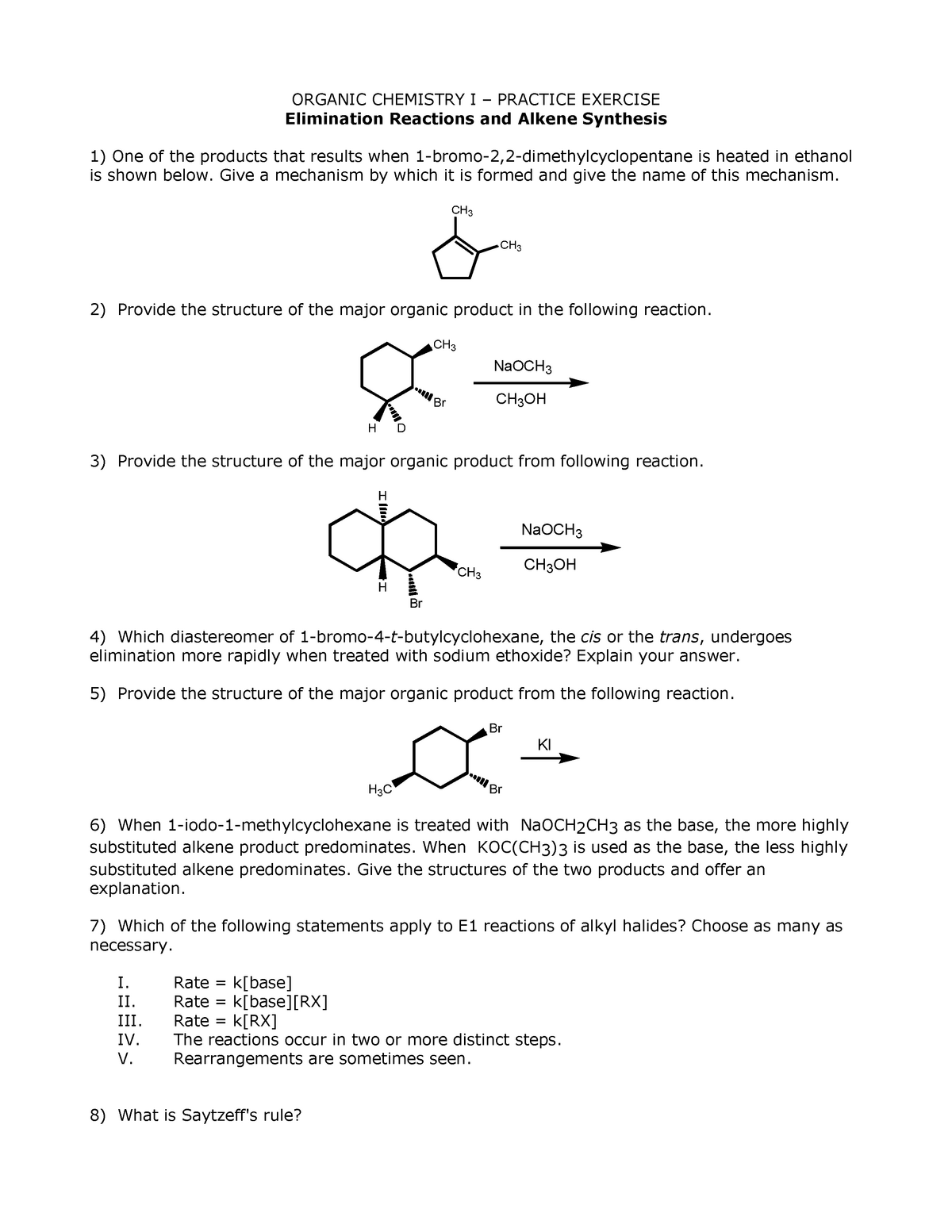
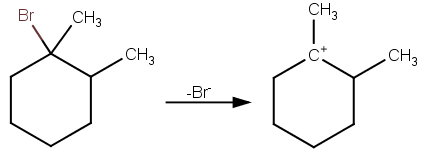
Step 1 The reaction between 1bromo1methylcyclohexane and in ethanol for an extended period of time produces three products The reaction follows first order nucleophilic substitution reaction(SN1) as well as unimolecular Elimination reaction (E1) Rate = k 1bromo1methylcyclohexane Step 2 Mechanism of the reaction isWhat product(s) are expected in the ethoxidepromoted betaelimination reaction of each of the following compounds?When (1R,2R)1bromo2methylcyclohexane reacts with sodium methoxide in presence of methanol, E2 elimination reaction occurs to form (R)3methylcycl See full answer below Become a member and
2
1-bromo-1-methylcyclohexane elimination
1-bromo-1-methylcyclohexane elimination-1bromo1methylcyclopentane in methanol as solvent Show all steps and all intermediates involved Use electronpushing arrows to indicate electron flow for each step Show both substitution and elimination productsThere are three common elimination mechanisms E 1, E 2 and E 1 c b E 1 and E 1 c b both imply that an ionic intermediate is formed first;




How To Determine The Stereochemistry After Elimination Of Anti 1 Bromo 2 Methylcyclohexane Chemistry Stack Exchange
Would it be a) 1methyl1cyclohexene b) a cyclohexane/ene with a double bond off of one C basically the cyclohexane shape with =CH2 off of it (I don't remember the actual name for it)? E1 elimination of 1bromo1methylcylclohexane using KOtBu?1), there are two mechanisms of elimination (E2 and E1) E2 mechanism — bimolecular elimination E1 mechanism — unimolecular elimination The E2 and E1 mechanisms differ in the timing of bond cleavage and bond formation, analogous to the S N 2and S N 1 mechanisms E2 and S N 2 reactions have some features in common, as do E1 and S N 1 reactions
The kinetics of dehydrobromination of 1bromo1methylcyclopentane in 10 protic and 31 aprotic solvents were studied by the verdazyl method v = kRBr, E1 mechanism The structure of 1bromo4methylcyclohexane is C1 and C4 are not chiral centres, so there are no optical isomers The molecule, however, exists as a pair of cis and trans isomers cis1bromo4methylcyclohexane and trans1bromo4methylcyclohexane In a cis1,4disubstutited cyclohexane, one substituent is always equatorial, and the other is always axialFor the second reaction, 1bromo1methylcyclohexane can be easily made via a bromination reaction to give a high yield of 1bromo1methylcyclohexane since bromination is selective for a tertiary C─H substitution
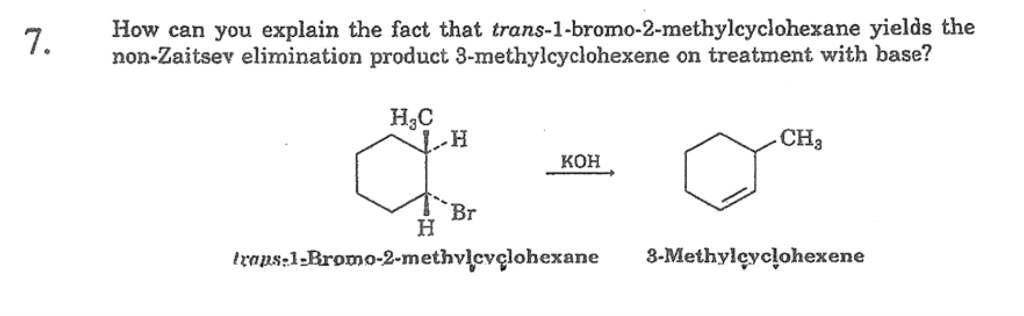
How can you explain the fact that trans1bromo2methylcyclohexane yields the nonZaitsev elimination product 3 methylcyclohexene on treatment with base?TheFateOfTime's Webcam Video from 0451 PM1 Predict the elimination products of the following reactions When two alkenes are possible, predict which one will be the major product Explain your answers, showing the degree of substitution of each double bond in the products cis1bromo2methylcyclohexane NaOCH3 CH3OH (b) trans1bromo2methylcyclohexane NaOCH3



2




Hypervalent Iodine Reagents Thiol Derivatization With A Tetrafluoroethoxy Coumarin Residue For Uv Absorbance Recognition Commare 17 Helvetica Chimica Acta Wiley Online Library
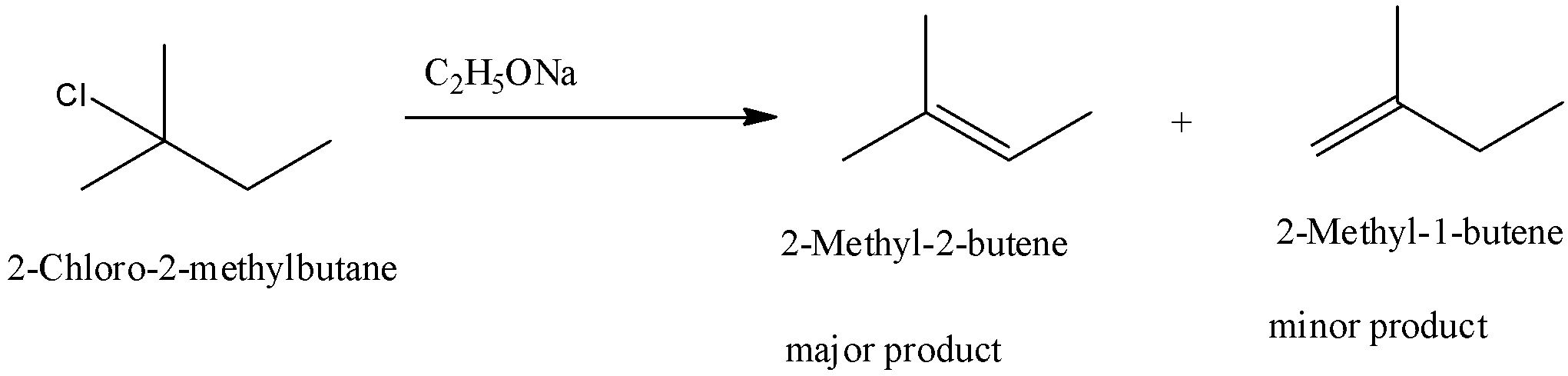
(EQUATION CAN'T COPY) Answer Elimination occurs to yield 3 MethylCy clohexane View Answer Topics Alkyl HalidesI) In 1bromo1methylcyclohexane compound, all atoms are equivalent Thus, dehydrohalogenation of this compound gives only one alkene ii) In 2chloro 2methyl butane compound, there are two different sets of equivalent atoms Thus, dehydrohalogenation of the compound yeilds two alkenes Saytzeff's rule implies that in dehydrohalgenation reaction, theSUBSTITUTION AND ELIMINATION REACTIONS OF ALKYL HALIDES SOLUTIONS TO PROBLEMS 61 (a) cis1Bromo2methylcyclohexane (b) cis1Bromo3methylcyclohexane (c) 2,3,4Trimethylheptane 62 (a) 3 (b) vinylic (c) 2 (d) aryl (e) 1 63 Substrate Nucleophile Leaving group CH 3 I CH 3CH 2 O CH 3 O CH 2CH 3 I (a) − − Nucleophile Substrate Leaving



Cis 1 Bromo 2 Methylcyclohexane Chair Conformation




Oneclass Design A Synthesis Of 1 Bromo 2 Methylcyclopentane From Methylenecyclopentane Br Part 1 Ou
1bromo1methylcyclohexane C7H13Br, synthesis, structure, density, melting point, boiling point CHEM What is the major product from an elimination reaction starting with 2bromo1methylcyclohexane?On a recent exam I was asked to identify the mechanism when 1bromo1methylcyclohexane was reacted with KOtBu in HOtbu, no other conditions were given (temp, concentration, etc)



8 4 The E2 Reaction And Cyclohexane Conformation Chemistry Libretexts




1 1 Draw The Structure Of S 1 Bromo 1 Chlorobutane 2 Draw The Structure Of 2r 3r 2 3 Dichloropentane
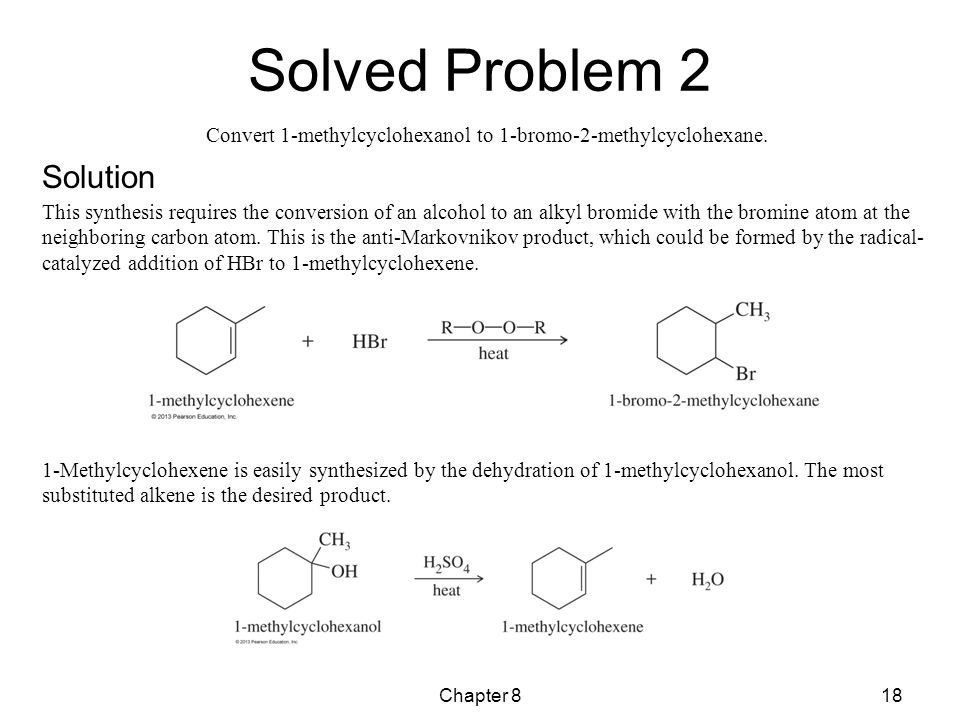
Ask Question Asked 4 years, 8 months ago Active 4 years, 4 months ago Viewed 970 times 1 $\begingroup$ The question is asking for the product and the answer is C {E2}$ elimination since that would require eliminating the methyl group Carry onIf no reaction occurs, draw the starting material When SN1 and E1 pathways compete, show both the substitution and the elimination products Do not include counter ions like Na or IConvert 1methylcyclohexanol to 1bromo2methylcyclohexane The twostep synthesis is summarized as follows Solved Problem 2 (Continued) Solution (Continued) Hydration of Alkenes 3 ways to add water to alkene (1st method here, Markovnikov with possibility of rearrangement)



Sodium Ethoxide




Predict All The Alkenes That Would Be Formed By Dehydrohalogenation Of The Following Halides With Sodium Ethoxide In Ethanol And Identify The Major Alkene I 1 Bromo 1 Methyl Cyclohexane Ii 2 Chloro 2 Methyl Butane Iii 2 2 3 Trimethyl 3
When 1bromo1methylcyclohexane is heated in ethanol for an extended period of time, three products result one ether and two alkenes Predict the products of this reaction, and propose a mechanism for their formation Predict which of the two alkenes is the major elimination productHow the following conversions can be carried out?The substituents present at 1 and 2 positions of a chair cyclohexane take an axial and an equatorial position in cis form, but both equatorial positions or both axial positions in the trans form Hence, the axial B r has an adjacent axial hydrogen at C 2 and (C 6) in cis form but only at C6 in trans Therefore, the former can give the more substituted 1 but the latter cannot



Would The Following Molecules React By Sn1 Or Sn2 Mechanism 1 Methyl 1 Bromo Cyclohexane And 2 Bromohexane Socratic




Which Is Are Correct Statements About The Minor Product
Both E1 and E2 reactions will form 1methylcyclohexene Both E1 and E2 reactions will form 3methylcyclohexeneOrganic Chemistry (8th Edition) answers to Chapter 6 Alkyl Halides Nucleophilic Substitution and Elimination Study Problems Page 280 643 b including work step by step written by community members like you Textbook Authors Wade Jr, L G, ISBN10 , ISBN13 , Publisher PearsonWhat products would be obtained when trans1chloro2methylcyclohexane undergoes an E1 reaction, and when it undergoes an E2 reaction?




Explain The Fact That Trans 1 Bromo 2 Methylcyvlohexane Yields The Non Zaitsev Elimination Product 3 Methylcyclohexene On Treatment With Koh Include The Structures Curved Arrows And A Few Words Study Com




1r 2r 1 Bromo 2 Methylcyclohexane E2 W Naoh To Form Alkene Ch7 Wp Ug 21 Youtube
(i) Propene to propan1ol (ii) Ethanol to but1yne (iii) 1bromopropane to 2bromopropane (iv) Toluene to benzyl alcohol (v) Benzene to 4bromonitrobenzene (vi) Benzyl alcohol to 2phenyl ethanoic acid (vii) Ethanol to propane nitrile (viii) Aniline to chlorobenzene (ix) 2chlorobutane to 3, 4dimethylhexane (x) 2methyl1propene toPredicted data is generated using the US Environmental Protection Agency's EPISuite™ Log OctanolWater Partition Coef (SRC) Log Kow (KOWWIN v167 estimate) = 390 Boiling Pt, Melting Pt, Vapor Pressure Estimations (MPBPWIN v142) Boiling Pt (deg C) (Adapted Stein & Brown method) Melting Pt (deg C) 1450 (Mean or Weighted MP) VP(mm Hg,25 deg C) 229E 1 implies the same carbocationic intermediate as in an S N 1 reaction The c b in E 1 c b means conjugate base, ie the first step is deprotonation of the compound to give an carbanionic intermediate



2




Identify Possible Products Of Dehydrohalogenation Of Trans 1 Bromo 2 Methylcyclohexane Study Com
Cis1bromo2methylcyclohexane MFCD Predicted ACD/Labs Predicted EPISuite Predicted ChemAxon Predicted data is generated using the ACD/Labs Percepta Platform PhysChem Module Density 13±01 g/cm 3 For example, trans2methyl1chlorocyclohexane reacts with alcoholic KOH at a much slower rate than does its cisisomer Furthermore, the product from elimination of the transisomer is 3methylcyclohexene (not predicted by the Zaitsev rule), whereas the cisisomer gives the predicted 1methylcyclohexene as the chief product These differencesMethyl14 C1methylI,4cyclohexadiene undergoes a dehydrogenation to toluene even at temperatures below 500°C This reaction takes place by a 1,4 hydrogen elimination (symmetry allowed) Besides toluene, another product in the pyrolyzate is methylcyclohexane, which is present at a lower level (< 10%) This compound is probably formed by



Solved Odium Ethoxide And Then What Would The Reaction Look Like From 1 Methylcyclohex 1 Ene Reacted With Molecular D2 In The Presence Of Platinum Course Hero



2
46 If the rate of reaction of 01 M sodium cyanide with 01M 1bromoethane is 14 x 104, what effect will an increase in NaCN concentration to 03 and alkyl bromide concentration to 02 have on the overall reaction rate?1Bromo1methylcyclopentane PubChem CID Structure Find Similar Structures Chemical Safety Laboratory Chemical Safety Summary (LCSS)1Bromo1methylcyclohexane C7H13Br CID structure, chemical names, physical and chemical properties, classification, patents, literature, biological




4 Why Does The Reaction Of Trans 1 Bromo 2 Methylcyclohexane Yield The Non Zaitsev Elimination Product 3 Methylcyclohexene On Treatment With Homeworklib




E2 Elimination Chem 215 Spring08 Openstax Cnx
1methylcyclohex1ene is formed by dehydrohalogenation of 1bromo1methylcyclohexane with alcoholic KOH If there is possibility of formation of more than one alkene, then major product is identified by C According to 1methylcyclohex1ene, during dehydrohalogenation, more substituted alkene is the major product The cisisomer undergoes elimination more readily The cisisomer undergoes elimination more readily Since the base is sodium ethoxide, the mechanism is E2 In an E2 elimination, the leaving group and the β hydrogen must go through an antiperiplanar transition state Now let's look at the structures of cis and trans1bromo4tbutylcyclohexaneWhat is the MAJOR ORGANIC product(s) of the reaction of 1bromo1methylcyclohexane with water?



John E Mc Murry Http Www Cengage Comchemistrymcmurry
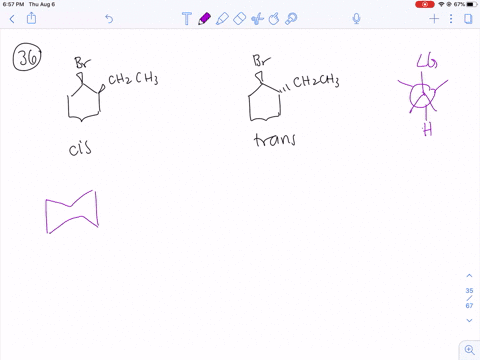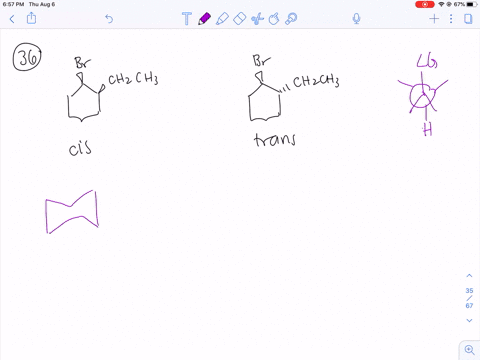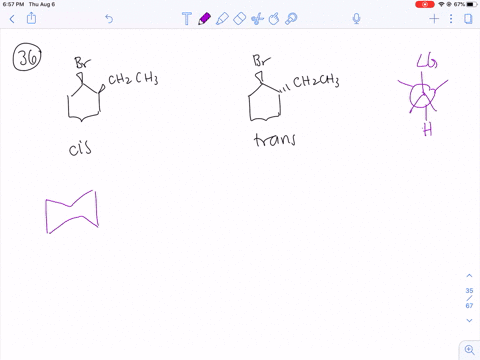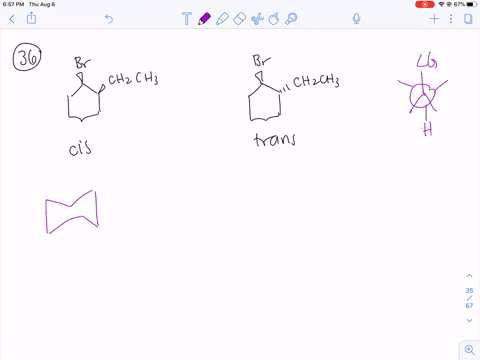



Solved How Can You Explain The Fact That Trans 1 Bromo 2 Methylcyclohexane Yields The Non Zaitsev Elimination Product 3 Methylcyclohexene On Treatment With Base Equation Can T Copy
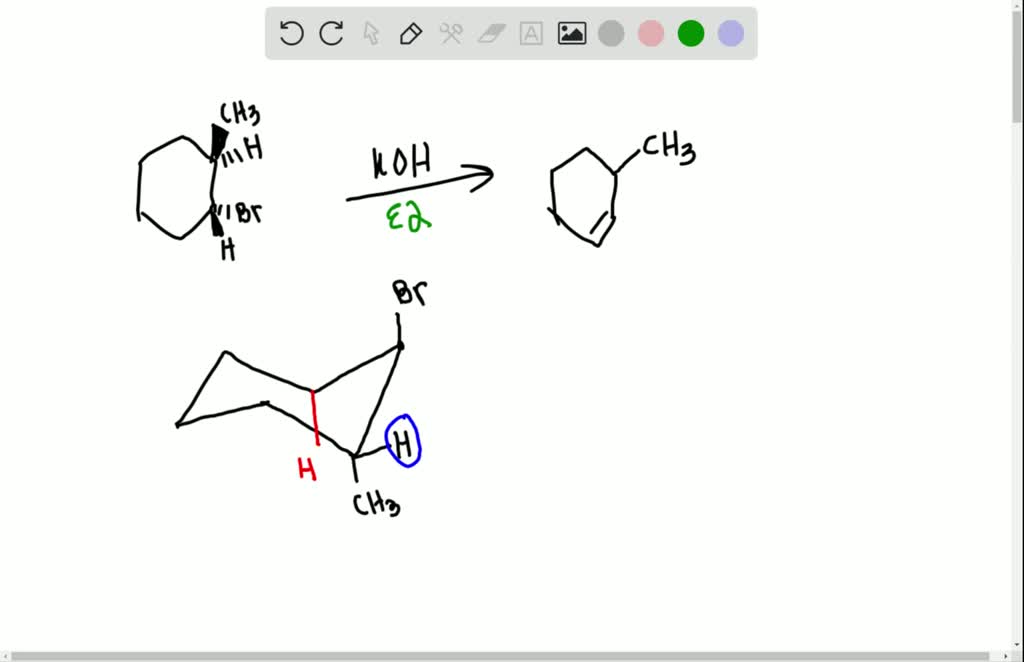
Would it be a) 1methyl1cyclohexene b) a cyclohexane/ene with a double bond off of one C basically the cyclohexane shape with =CH2 off of it (I don't Organic chemistry4) Which diastereomer of 1bromo4tbutylcyclohexane, the cis or the trans, undergoes elimination more rapidly when treated with sodium ethoxide?How can you explain the fact that trans1Bromo2methylcyclohexane yields the nonZaitsev elimination product 3methylcyclohexane on treatment with base close Start your trial now!



08 Alkesynth



Predict All The Alkenes That Would Be Formed By Dehydrohalogenation Class 11 Chemistry Cbse
(a)2bromo2, 3dimethylbutane ( b)1chloro1methylcyclohexaneSUBSTITUTION AND ELIMINATION REACTIONS OF ALKYL HALIDES SOLUTIONS TO PROBLEMS 61 (a) cis1Bromo2methylcyclohexane (b) cis1Bromo3methylcyclohexane (c) 2,3,4Trimethylheptane 62 (a) 3 (b) vinylic (c) 2 (d) aryl (e) 1 63 Substrate Nucleophile Leaving group CH 3 I CH 3CH 2 O CH 3 O CH 2CH 3 I (a) − − Nucleophile LeavingSubstrateExplain your answer 5) Provide the structure of the major organic product from the following reaction Br H3C Br KI 6) When 1iodo1methylcyclohexane is treated with NaOCH2CH3 as the base, the more




Alkyl Halides Nucleophilic Substitution And Elimination Ppt Video Online Download
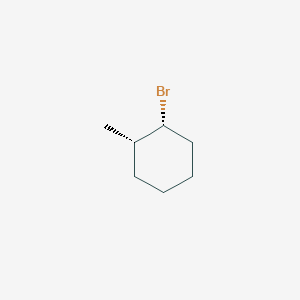



Cis 1 Bromo 2 Methylcyclohexane C7h13br Pubchem
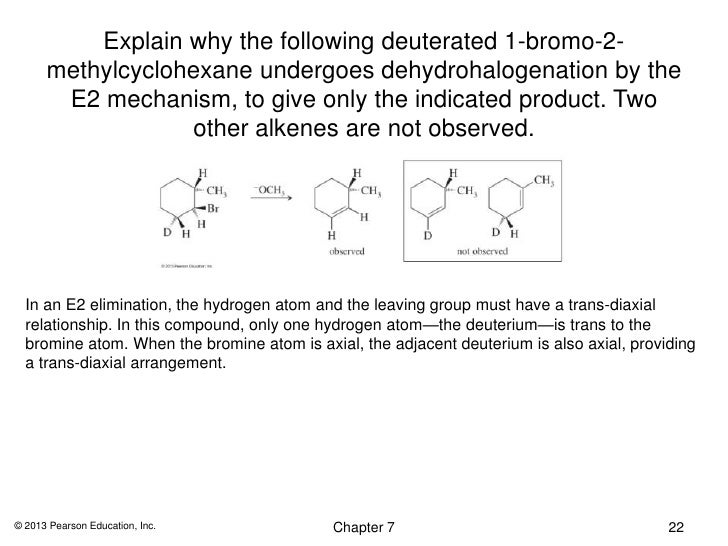
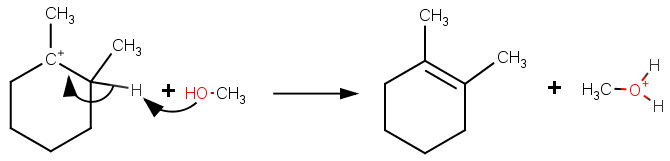
NIST/TRC Web Thermo Tables (WTT) NIST Standard Reference Subscription Database 3 Professional Edition Version 2121Pro This web application provides access to a collection of critically evaluated thermodynamic property data for pure compounds with a primary focus on organics These data were generated through dynamic data analysis, as implemented in the NIST 1 Answer to Explain why the following deuterated 1bromo2methylcyclohexane undergoes dehydrohalogenation by the E2 mechanism, to give only the indicated product Two other alkenes are not observedC) 3methyl1cyclohexene d) 4methyl1cyclohexene



What Will Be The Product In The Reaction Between 1 Methylcyclohexene And Hcl Quora



2
711 (a) Anti coplanar elimination can occur in two ways with the cis isomer H H H Br CH3 H B• • (b) (a) (a) (b) cis1Bromo2methylcyclohexane CH3 CH3 (major product) (b) Anti coplanar elimination can occur in only one way with the trans isomer H H H H Br B•• CH3 trans1Bromo2methylcyclohexane CH3 712 (a) 3CH OH O CH 3 (1) (2Treating 1bromo1methylcyclohexane with CH3OH at room temperature would yield I know the answer is D) All of these I would like a thorough explanation as to why I thought it can only undergo SN1 making it answer A) Question Treating 1bromo1methylcyclohexane with CH3OH at room temperature would yield I know the answer is D) All ofTheFateOfTime's Webcam Video from 0331 PM
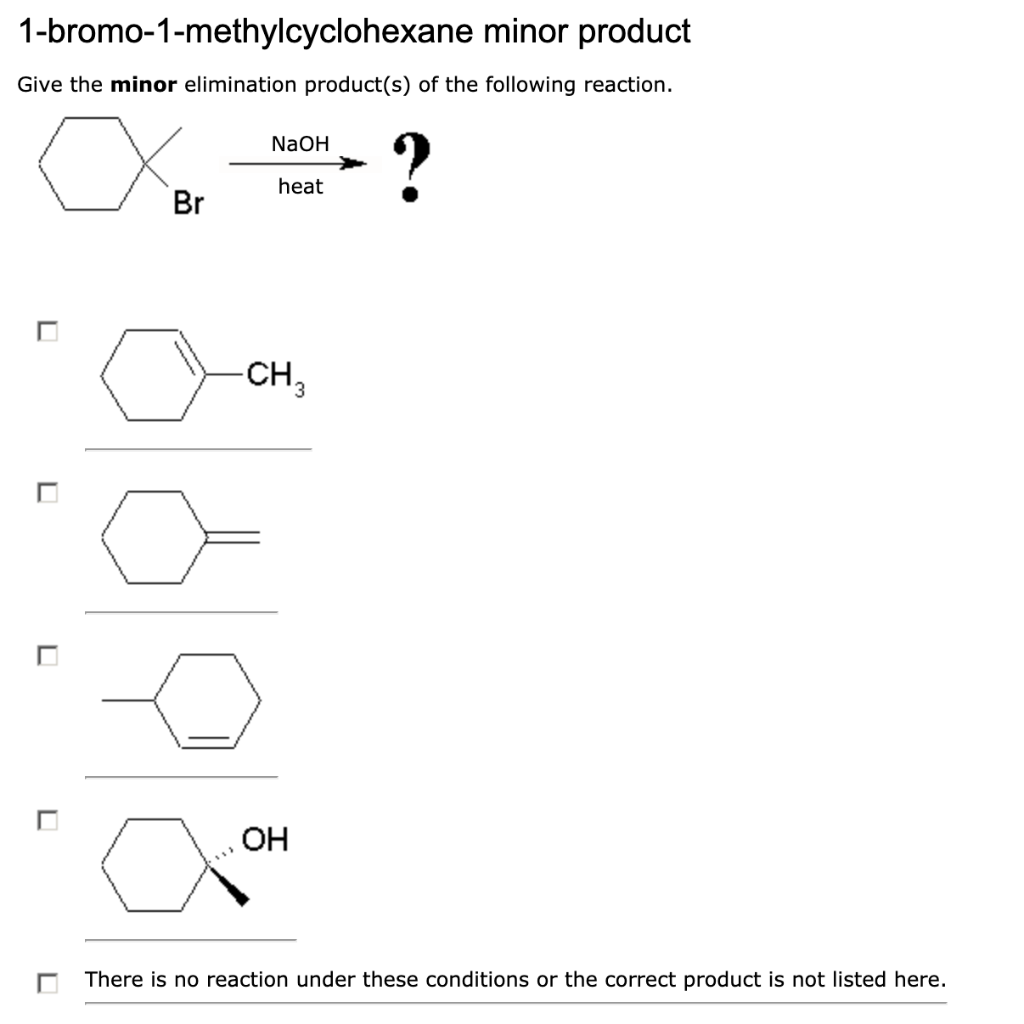



Solved 1 Bromo 1 Methylcyclohexane Minor Product Give The Chegg Com



Exam 3 Answer Key
How to determine the stereochemistry after elimination of anti1bromo2methylcyclohexane?Cis1bromo2methylcyclohexane with KOH in ethanol to form 1methylcyclohexene Use a chair form and electronpushing arrows to show the stereochemistry of the mechanism 12 (10 points) Write a complete mechanism for the solvolysis of tertbutyl chloride in methanol Show all steps and use electronpushing arrowsFor each reaction, decide whether substitution or elimination (or both) is possible, and predict the products you expect Part A Draw the major product from the following reaction 1bromo1methylcyclohexane in acetone Draw the molecule on the canvas by choosing buttons from the Tools (for bonds and charges), Atoms, and Templates toolbars




1 Bromo 1 Methyl Cycloheptane Reacts With Koh Draw The Stru Clutch Prep




Compound A Reacts With One Equivalent Of H2 In The Presence Of A Catalyst To Give Methylcyclohexane Brainly Com
AnswerIt will form 1methyl1cyclohexeneExplanationAs it is a elimination reaction because ethanol and sodium ethoxide are bulkier group which is not easy to atharvashuklaobra atharvashuklaobra Chemistry Secondary School answered What happens when 1bromo1methylcyclohexane react with ethanol and sodium ethoxide?What is the major product from an elimination reaction starting with 2bromo1methylcyclohexane?Draw the substitution and elimination products for the following reactions, showing the configuration of each producta trans 1 chloro 2 methylcyclohexane C H 3 O −b cis1 chloro 2 methylcyclohexane C H 3 O −c 1 chloro 1 methylcyclohexane C H 3 Od 1 chloro 1 methylcyclohexane C H 3 O H




When 1 Bromo 1 Methylcyclohexane Is Heated In Studysoup Sabri Wyatt Pdf Livres Gratuits




How To Determine The Stereochemistry After Elimination Of Anti 1 Bromo 2 Methylcyclohexane Chemistry Stack Exchange




2 10 Points Explain Why The Following Deuterated 1 Bromo 2 Methylcyclohexane Undergoes Dehydrohalogenation By Th Homeworklib
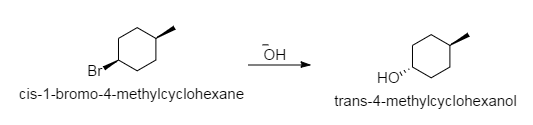



Draw The Substitution Product Formed When Cis 1 Bromo 4 Meth Quizlet




Solution Identify Possible Product S Of Clutch Prep




Elimination Reactions Of Cis And Trans 1 Bromo 2 Methylcyclohexanes With Naoet In Etoh Can Give The Same Or Different Main Product 1 Methylcyclohexene 1 Or 3 Methylcyclohexene 2 Which Of A D Indicates The Main Products




Elimination Mechanisms Organic Chemistry
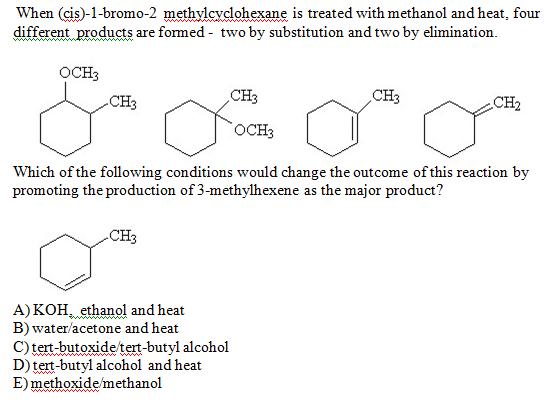



Solved When Cis 1 Bromo 2 Methylcyclohexane Is Treated Chegg Com




E2 Elimination Anti Periplanar Beta Hydrogen Chemistry Stack Exchange
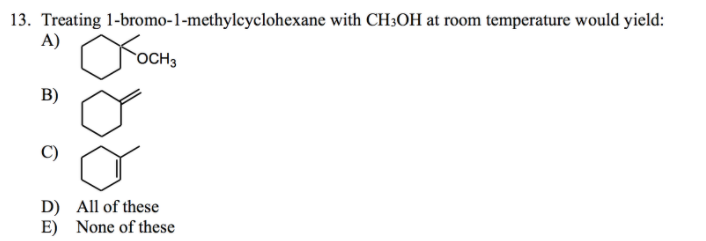



13 Treating 1 Bromo 1 Methylcyclohexane With Ch3oh At Room Temperature Would Yield A Och B C D E All Of These None Of These Wegglab




The Clinical Use Of Nor Epinephrine In The Treatment Of Shock Accompanying Myocardial Infarction And Other Conditions Nejm



Ppt Elimination Reactions Powerpoint Presentation Free Download Id




Structure And Synthesis Of Alkenes Ppt Video Online Download



Exam 3 Answer Key




Hbr In H3coh 1 Bromo 1 Methylcyclopentane 85 1 Methylcyclopentyl Acetate 15 Propose A Reaction Mechanism For The Formation Of Homeworklib



Elimination Reactions Alkene Synthesis Worksheet With Answer Key Organic Chemistry I Practice Studocu




How To Determine The Stereochemistry After Elimination Of Anti 1 Bromo 2 Methylcyclohexane Chemistry Stack Exchange



Solved Of Cis 1 Bromo 2 Methylcyclohexane And Trans 1 Bromo 2 Methyl Cyclohexane Also Which Alkenes Are Formed From Each Alkyl Halide Under E2 C Course Hero



Final Exam Answer Key



What Will Happen If I Heat A Solution Of 1 Bromo 1 2 Dimethylcyclohexane In Methanol Socratic



Solved How Can You Explain The Fact That Trans 1 Bromo 2 Methylcyclohexane Yields The Non Zaitsev Elimination Product 3 Methylcyclohexene On Treatment With Base Equation Can T Copy




Solved Compound A C7h13br Is A Tertiary Bromide On Treatment With Sodium Ethoxide In Ethanol A Is Convert Into B C7h12 Ozonolysis Of B Gives C As The Only Product A



Organic Syntheses Procedure




Methylcyclopentane An Overview Sciencedirect Topics



What Will Happen If I Heat A Solution Of 1 Bromo 1 2 Dimethylcyclohexane In Methanol Socratic




Solved Predict All The Alkenes That Would Be Formed By Dehydrohalogenation Of The Following Halides With Sodium Ethoxide In Ethanol And Identify The Major Alkene I 1 Bromo 1 Methylcyclohexane




Solution Identify Possible Product S Of Clutch Prep



Quiz 8
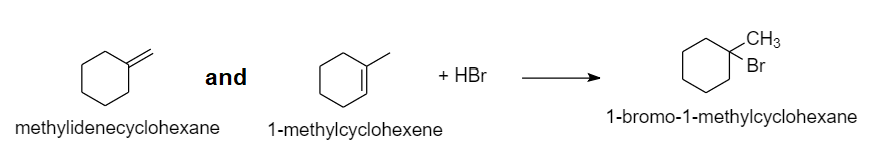



A Identify Two Alkenes That React With Hbr To Form 1 Bromo Quizlet
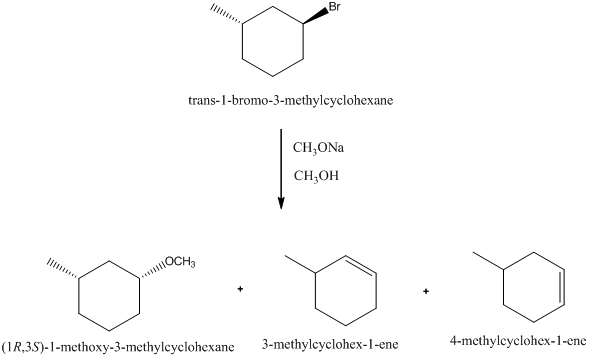



Solved Chapter 9 Problem 4p Solution Organic Chemistry 6th Edition Chegg Com



2




8 4 The E2 Reaction And Cyclohexane Conformation Chemistry Libretexts




1 Draw The Structure Of S 1 Bromo 1 Chlorobutane Ppt Video Online Download




Write The Structure Of The Alkene Formed By Dehydrohalogenation Of 1 Bromo 1 Methylcyclohexane With Brainly In
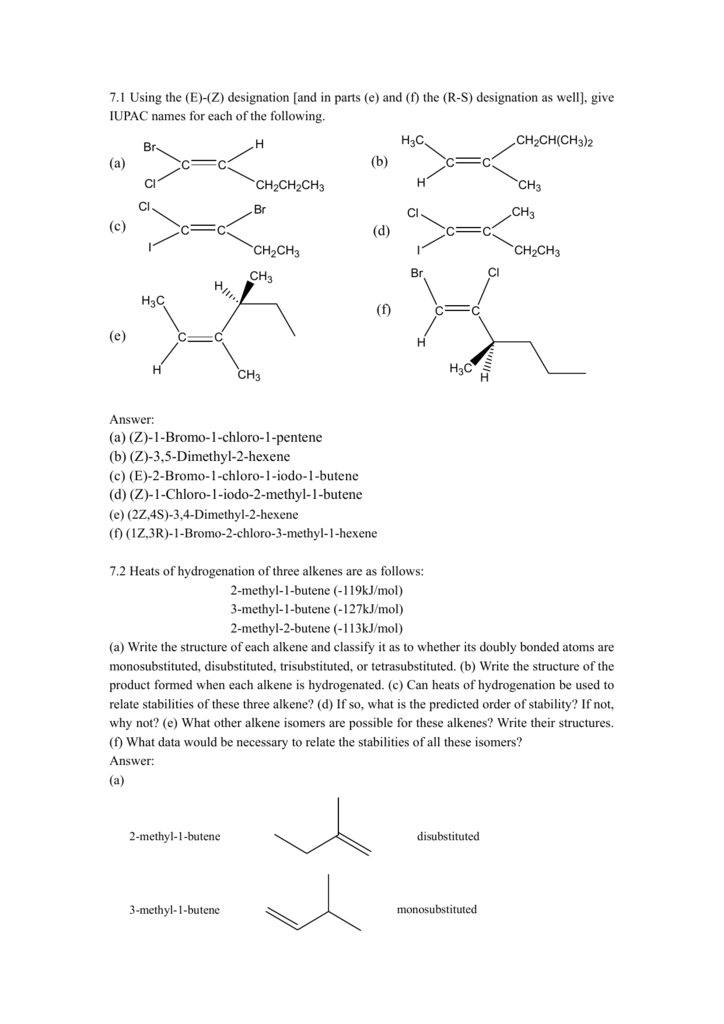



Z 1 Bromo 1 Chloro 1



2




11 8 19 The Cis And Trans Isomers Of 1 Bromo 2 Methylcyclohexane Are Shown Below One Of These Isomers Undergoes E2 Homeworklib




Chapter 6 Tb Flashcards Quizlet



2



2



2
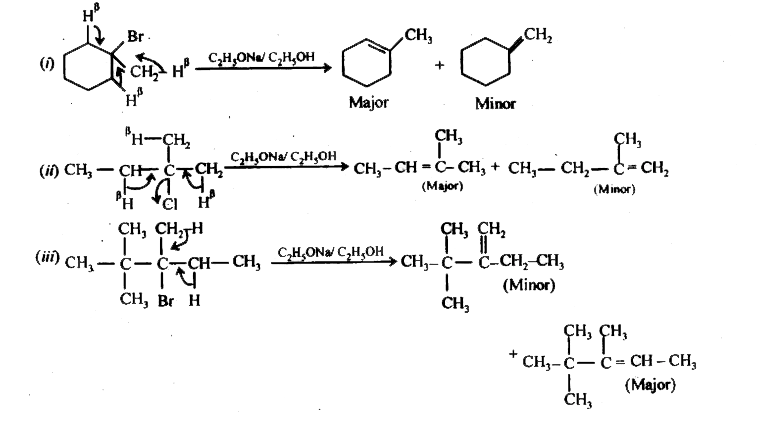



When 1 Bromo 1 Methylcyclohexane Is Heated In Ethanol For An Extended Period Of Time Three Products Result One Either And Two Alkenes Predict The Products Of This Reaction And Propose Mechanism For



3 Bromo 1 Methylcyclohex 1 Ene C7h11br Pubchem



3 Bromo 1 Methylcyclohexane 1 Carboxylic Acid C8h13bro2 Pubchem



Solved How Can You Explain The Fact That Chegg Com




Write The Structure Of The Alkene Formed By Dehydrohalogenation Of 1 Bromo 1 Methylcyclohexane With Alcoholic Koh
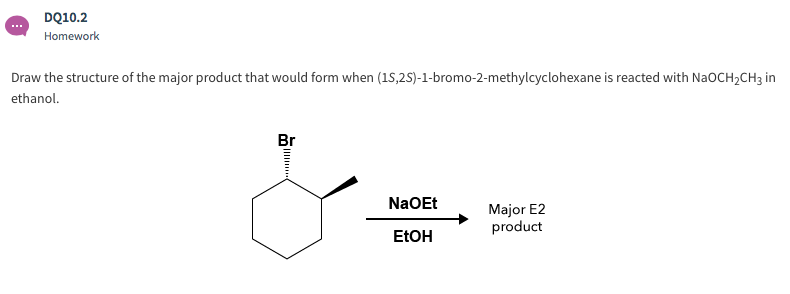



Solved Dq10 2 Homework Draw The Structure Of The Major Chegg Com



Final Exam Answer Key




When 1 Bromo 1 Methylcyclohexane Is Heated In Ethanol For An Extended Period Of Time Three Products Result One Either And Two Alkenes Predict The Products Of This Reaction And Propose Mechanism For
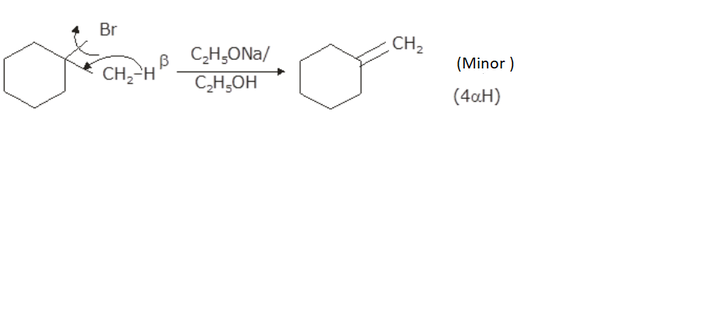



1 Bromo 1 Methylcyclohexane Predict All The Alkenes That Would Be Formed By



Regioselectivity In Alkene Addition Reactions Master Organic Chemistry
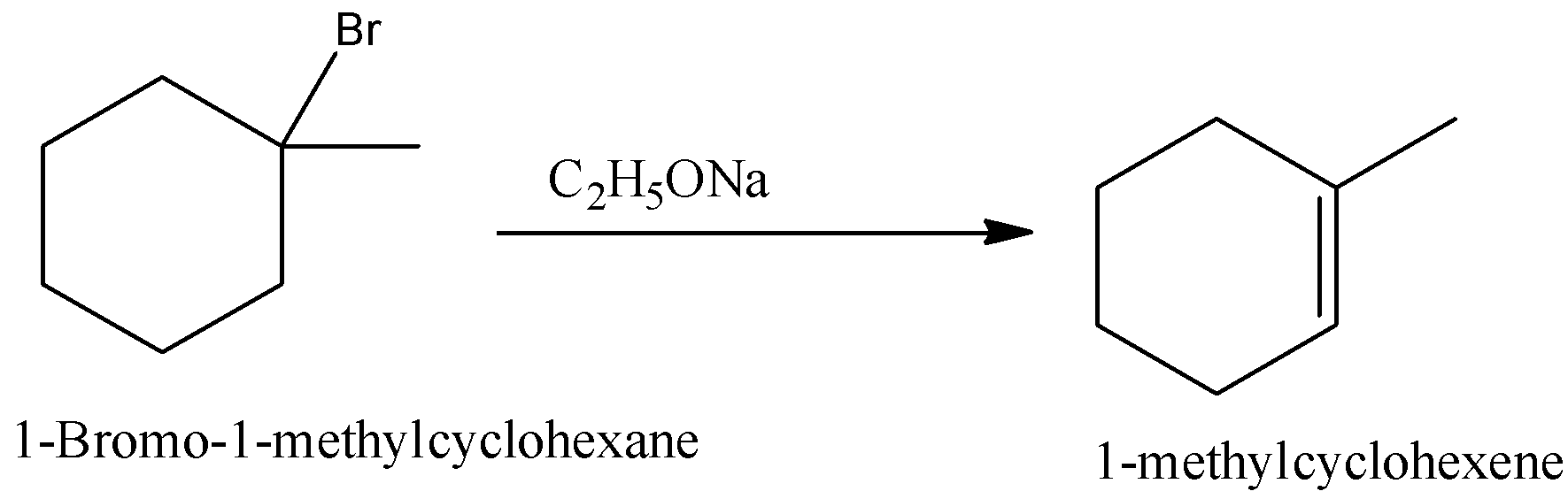



Predict All The Alkenes That Would Be Formed By Dehydrohalogenation Class 11 Chemistry Cbse




Solved Explain Why The Following Deuterated 1 Bromo 2 Methylcyclohexane 1 Answer Transtutors



1 Bromo 1 Methylcyclohexane H2o



Sn1 Reaction



Che 311 Organic Chemistry I Ppt Video Online Download
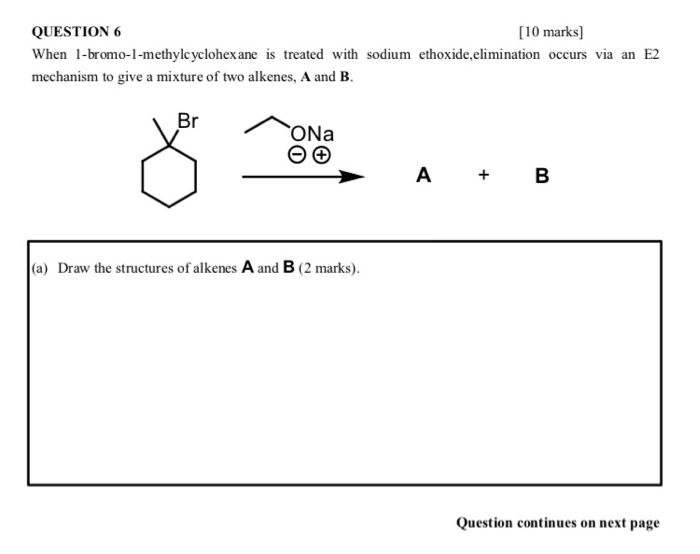



Solved Question 6 When 1 Bromo 1 Methylcyclohexane Is Chegg Com
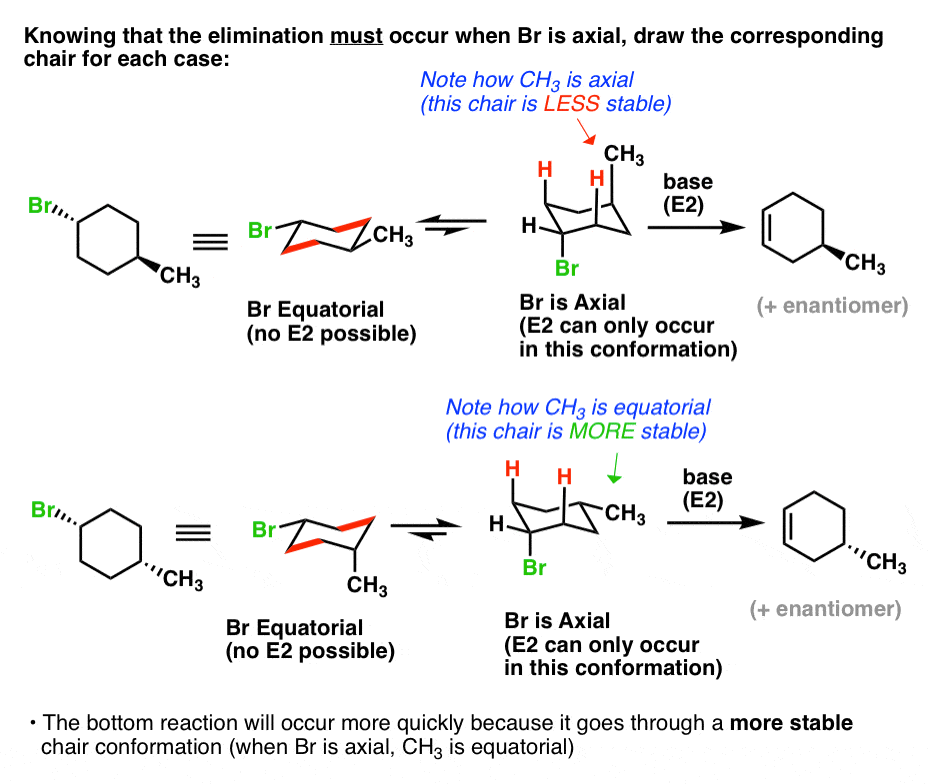



Antiperiplanar Relationships The E2 Reaction And Cyclohexane Rings




E2 Elimination Anti Periplanar Beta Hydrogen Chemistry Stack Exchange



2



2




Oneclass Predict And Draw The Major Elimination Products Of The Following Reactions Cis 1 Bromo 2 M
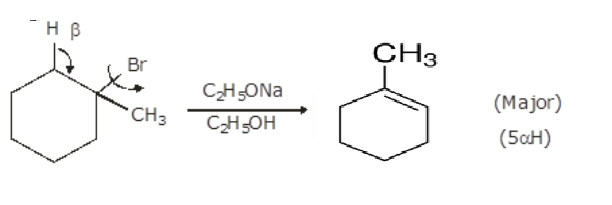



1 Bromo 1 Methylcyclohexane Predict All The Alkenes That Would Be Formed By



2




Solution Identify Possible Product S Of Organic Chem
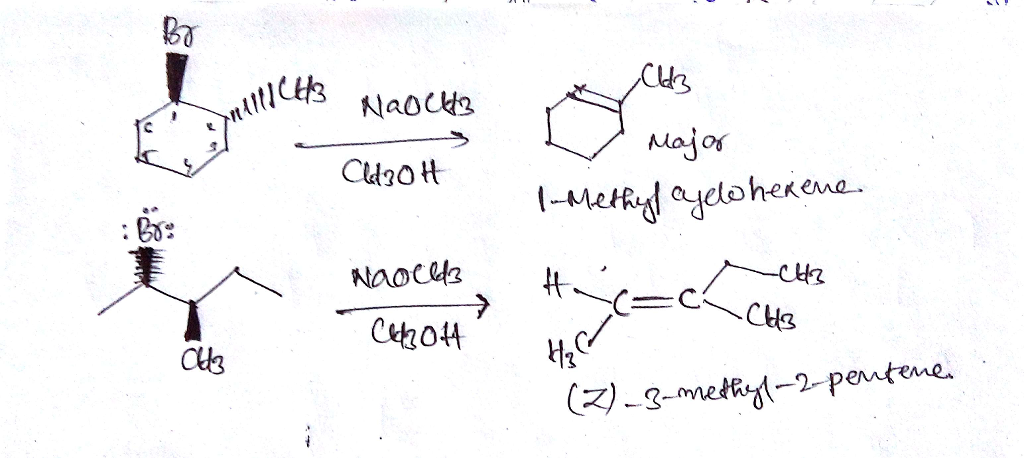



Question Answer When 1r 2r 1 Bromo 2 Methylcyclohexane Is Heated With Sodium Methoxide In Methanol Grand Paper Writers




When Trans 1 Bromo 2 Methylcyclohexane Is Treated With Strong Base The Major Product Is 3 Methylcyclohexene This Product Is The Non Zaitsev Elimination Product Explain These Experimental Results Study Com



Free Ncert Solutions For 12th Class Chemistry Haloalkanes And Haloarenes ह ल एल क न तथ ह ल एर न स Studyadda Com



1 Bromo 2 Methylcyclohexane In Stock



Alkyl Halide Reactivity




1 Bromopropane An Overview Sciencedirect Topics




Predict And Draw The Structure Of The Products For The Reactions Below A 1 Bromo 1 Methylcyclohexane Triethylamine Rightarrow B 2 Bromo 2 Butane Sodium Methoxide Rightarrow Study Com




1r 2r 1 Bromo 2 Methylcyclohexane E2 Rxn W T Butok Ch7 Wp Ug 23 Youtube
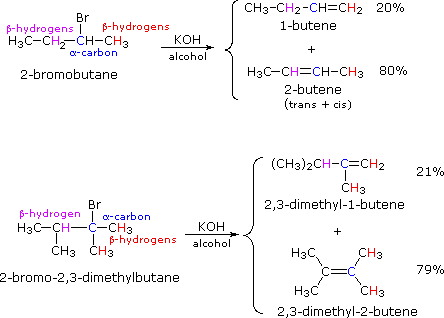



Alkyl Halide Reactivity



0 件のコメント:
コメントを投稿